NORTHERN BLOTTING
Northern Blotting is a technique used for the study of gene expression. It is done by detection of particular RNA (or isolated mRNA). mRNA is generally represented as 5% of the overall RNA sequence. This method reveals the identity, number, activity, and size of the particular gene. This blotting technique can also be used for the growth of a tissue or organism. In different stages of differentiation and morphogenesis the abundance of an RNA changes and this can be identified using this technique. It also aids in the identification of abnormal, diseased or infected condition at the molecular level. The northern blot technique was developed in 1977 by James Alwine, David Kemp and George Stank at Stanford University. The technique got its name due to the similarity of the process with Southern blotting. The primary difference between these two techniques is that northern blotting concerns only about RNA.
Principle
As all normal blotting technique, northern blotting starts with the electrophoresis to separate RNA samples by size. Electrophoresis separates the RNA molecules based on the charge of the nucleic acids. The charge in the nucleic acids is proportional to the size of the nucleic acid sequence. Thus the electrophoresis membrane separates the Nucleic acid sequence according to the size of the RNA sequence. In cases where our target sequence is an mRNA, the sample can be isolated through oligo cellulose chromatographic techniques, as mRNA are characterized by the poly(A)-tail. Since gel molecules are fragile in nature, the separated sequences are transferred to the nylon membranes. The selection of nylon membrane is contributed to the factor that nucleic acids are negatively charged in nature. Once the RNA molecules are transferred it is immobilized by covalent linkage. The probe is then added, the probe can be complementary an ss DNA sequence. Formamide is generally used as a blotting buffer as it reduces the annealing temperature.
Procedure
The tissue or culture sample collected is first homogenized. The samples may be representative of different types of culture for comparison or it can be for the study of different stages of growth inside the culture.
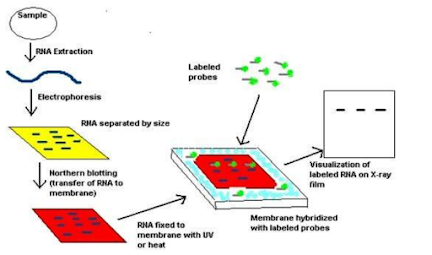
1. The RNA sequence is separated in the electrophoresis unit an agarose gel is used for the purpose of the nucleic acid separation.
2. Now the separated RNA sequence is transferred to the nylon membrane. This is done by two mechanisms capillary action and the ionic interaction.
3. The transfer operation is done by keeping the gel in the following order. First, the agarose gel is placed on the bottom of the stack, followed by the blotting membrane. On top of these paper towels a mild weight (glass plate) is placed. The entire setup is kept in a beaker containing transfer buffer.
4. RNA transferred to the nylon membrane is then fixed using UV radiation.
5. The fixed nylon membrane is then mixed with probes. The probes are specifically designed for the gene of interest, so that they will hybridize with RNA sequences on the blot corresponding to the sequence of interest.
6. The blot membrane is washed to remove unwanted probe
7. Labeled probe is detected by chemiluminescence or autoradiography. The result will be dark bands in x ray film.
Applications of Northern Blotting
1. Detection of Specific mRNA in sample
2. In disease diagnosis
3. In gene expression studies
4. In the screening of recombinants by detecting the mRNA produced by the transgene