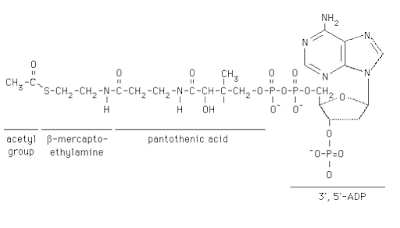
Coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, and it has significant role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. Around 4% of cellular enzymes use it (or a thioester) as a substrate,and all genomes sequenced to date encode enzymes that use coenzyme A as a substrate.
Discovery
Coenzyme A was identified by Fritz Lipmann in 1946.Its
structure was determined during the early 1950s at the Lister Institute,
London, together by Lipmann and other workers at Harvard Medical School and
Massachusetts General Hospital. Lipmann initially intended to study acetyl
transfer in animals, and from these experiments he noticed a unique factor that
was not present in enzyme extracts but was evident in all organs of the
animals. He was able to isolate and purify the factor from pig liver and
discovered that its function was related to a coenzyme that was active in
choline acetylation. The coenzyme was named coenzyme A to stand for
"activation of acetate". In 1953, Fritz Lipmann won the Nobel Prize
in Physiology or Medicine "for his discovery of co-enzyme A and its
importance for intermediary metabolism".
Structure
In humans, CoA biosynthesis requires cysteine, vitamin B5 (pantothenate ), and adenosine triphosphate (ATP). In its acetyl form, coenzyme A is a highly versatile molecule, serving metabolic functions in both the anabolic and catabolic pathways. Acetyl-CoA is utilised in the post-translational regulation and allosteric regulation of pyruvate dehydrogenase and carboxylase to maintain and support the partition of pyruvate synthesis and degradation.
Chemical formula- C21H36N7O16P3S
Molar mass- 767.535
Biosynthesis
In all living organisms, coenzyme A is synthesized in a
five-step process that requires four molecules of ATP, pantothenate and
cysteine.
1. Pantothenate (vitamin B5 ) is phosphorylated to
4′-phosphopantothenate by the enzyme pantothenate kinase (PanK; CoaA; CoaX).
This is the committed step in CoA biosynthesis and requires ATP.
2. A cysteine is
added to 4′-phosphopantothenate by the enzyme phosphopantothenoylcysteine
synthetase (PPCS; CoaB) to form 4'-phosphoN-pantothenoylcysteine (PPC). This
step is coupled with ATP hydrolysis.
3. PPC is
decarboxylated to 4′- phosphopantetheine by phosphopantothenoylcysteine
decarboxylase (PPC-DC; CoaC)
4. 4′-Phosphopantetheine is adenylated (or more properly,
AMPylated) to form dephospho-CoA by the enzyme phosphopantetheine adenylyl
transferase (PPAT; CoaD)
5. Finally, dephospho-CoA is phosphorylated to coenzyme A by
the enzyme dephosphocoenzyme A kinase (DPCK; CoaE). This final step requires
ATP
Alternate pathway
When in body coA is not produced then, coenzyme A needs to
be provided from an external source, such as food, in order to produce 4′- phosphopantetheine.
Ectonucleotide pyrophosphates (ENPP)
degrade coenzyme A to 4′-phosphopantetheine, a stable molecule in organisms.
Acyl carrier proteins (ACP) (such as ACP synthase and ACP degradation) are also
used to produce 4′-phosphopantetheine. This pathways allows for
4′-phosphopantetheine to be replenished in the cell and allows for the
conversion to coenzyme A through enzymes, PPAT and PPCK.
Commercial production
Coenzyme A is commercially produced via extraction from
yeast, however this is an inefficient process (yields about 25mg/kg) resulting
in an expensive product. Various ways of producing CoA synthetically, or
semi-synthetically have been investigated although none are currently operating
at an industrial scale.
Function
Fatty acid synthesis
Since coenzyme A have a thiol group, it can react with carboxylic
acids to form thioesters, thus functioning as an acyl group carrier. It facilitate
in transferring fatty acids from the
cytoplasm to mitochondria. A molecule of coenzyme A carrying an acyl group is
also referred to as acyl-CoA. When it is not attached to an acyl group, it is
usually referred to as 'CoASH' or 'HSCoA'. This process facilitates the
production of fatty acids in cells, which are essential in cell membrane structure.
Coenzyme A is also the source of the phosphopantetheine group that is added as
a prosthetic group to proteins such as acyl carrier protein and
formyltetrahydrofolate dehydrogenase.
Energy production
Coenzyme A is one of five crucial coenzymes that are
necessary in the reaction mechanism of the citric acid cycle. Its
acetyl-coenzyme A form is the primary input in the citric acid cycle and is
obtained from glycolysis, amino acid metabolism, and fatty acid beta oxidation.
This process is the body's primary catabolic pathway and is essential in
breaking down the building blocks of the cell such as carbohydrates, amino
acids, and lipids.
Regulation
When there is excess glucose, coenzyme A is used in the
cytosol for synthesis of fatty acids. This process is implemented by regulation
of acetyl-CoA carboxylase, which catalyzes the committed step in fatty acid
synthesis. Insulin stimulates acetyl-CoA carboxylase, while epinephrine and
glucagon inhibit its activity. During cell starvation, coenzyme A is
synthesized and transports fatty acids in the cytosol to the mitochondria.
Here, acetyl-CoA is generated for oxidation and energy production. In the citric acid cycle, coenzyme A works as
an allosteric regulator in the stimulation of the enzyme pyruvate
dehydrogenase. New research has found that protein CoAlation plays an important
role in regulation of the oxidative stress response. Protein CoAlation plays a
similar role to S-glutathionylation in the cell, and prevents the irreversible
oxidation of the thiol group in cysteine on the surface of cellular proteins,
while also directly regulating enzymatic activity in response to oxidative or
metabolic stress.